This page lists the activities presented by the project teams at the C5 virtual meeting in Greece, whose topic was teaching and learning with experiments.
Each team presented at least 2 activities, one in the Maths and Science category and one in Art and Humanities category.
Maths and Science
SPAIN:
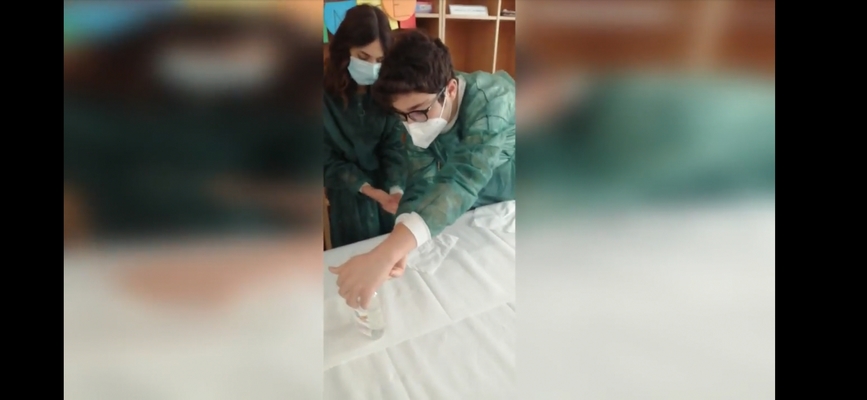
ITALY: "Clean hands" Let's protect ourselves : it's easy!
Dirty hands? The experiment that demonstrates what happens when you don't wash your hands well. How important is it to wash your hands? Especially in a period like the one we are experiencing all experts agree that hand washing is the main prevention measure against coronavirus. But how to explain it to the students? With a little experiment.
The experiment highlights the various levels of hand cleaning:dirty hands, hands washed with soap and water, hands on which hand sanitizer was used.


GREECE:
ROMANIA:
Due to pandemic conditions, we had to adapt to meeting online, which was undoubtedly a challenge that we coped with successfully. Besides being organized alongside our project friends, these virtual meetings are a confirmation that the educational systems everywhere are prepared to adapt and face any difficulty we may encounter. This mobility should have been hosted by the Greek school 5o Gymnasio in Volos. Teachers, students and coordinators alike did their best to manage things as smoothly as possible for everybody involved.
The Romanian team prepared two presentations for this meeting. The first one dealt with experiments, with Romanian scientists and their famous inventions and achievements, whereas the second had a more cultural overtone: the beauty of Romanian traditional dances.
Although nothing and nobody can minimize the relevance of face- to -face communication and interaction, we believe that this is the most valuable lesson in this case: adjusting to changing conditions is possible if we all work together for a common goal.




PORTUGAL:
Our Greek presentations wanted to know the Chemical behavior of the Alkali and Alkaline Earth Metals.
We have presented the Periodic Table (PT) which is organized in 7 horizontal lines (Period) and divided into columns - 18 groups.
The first two are of interest to us:
1st Group - Alkali Metals
2nd Group - Alkaline Earth Metals
We also thought that the chemical elements have similar properties placed in the same groups as PT.
This is what we wanted to prove in our school Lab.
We also wanted to prove that the reactivity is related to how easily atoms lose electrons to become ions.
We can also say that the reactivity of the elements is directly linked to the atomic ray.
Of the elements we studied (Li, Na, Mg and Ca). Sodium is the most reactive.
We went to our school Lab to test them all….
Lab material:
- spatula; - tub; - petri dish; - phenolphthalein; - water;
Procedure
- We filled the petri box with water,
- With the help of a spatula we put a little lithium in the water;
- We added a few drops of phenolphthalein at the end of the reaction.
- We recorded the observations.
- We carried out this procedure with the other substances (sodium, magnesium, and calcium)
- During the reaction of calcium with water, we placed a funnel facing downwards over the reaction;
- We approached a lit match at the exit of the funnel.
- We observed what happened.
Observations
When lithium and sodium were placed in the water, they became a sphere that swirled on the surface of the water.
During the reaction, we observed the release of a gas.
The gas ignited when we approached a flame.
When phenolphthalein was added to each of the solutions, they acquired a pink color.
The reaction of magnesium with water took the longest.
The reaction of sodium with water was faster than the reaction of lithium.
Conclusions
We can conclude that during the reaction of these metals with water a hydroxide was formed, Because when we added phenolphthalein Its color changed from colorless to pink, which indicates the presence of a basic solution.
We also concluded that if the gas released was hydrogen, because when we approached the flame with the gas, it reacted violently by igniting itself. The flame test - proved the release of hydrogen.
The reaction of sodium with water was more violent than that of lithium with water. Therefore, we can conclude that the reactivity increases through the group since it is easier to yield 1 electron that is farther from the nucleus.
As the reaction of magnesium with water was slower than that of calcium, once again we can conclude that the reactivity increases throughout the group.
The reactions of the elements of the first group were more violent than those of the elements of the second group, so we conclude that Alkali Metals are more reactive than Alkaline Earth Metals.
Reactivity increases throughout the group and decreases over the period.




C5-Chemistry.pptx
ART and HUMANITIES
SPAIN:
ITALY:
Dante's voyage; from fear to happines
The learning unit was presented by our students of secondary school to the children of the fifth grade of our Primary School during an online meeting based on the continuity between the various school orders in the occasion of Dante’s day.
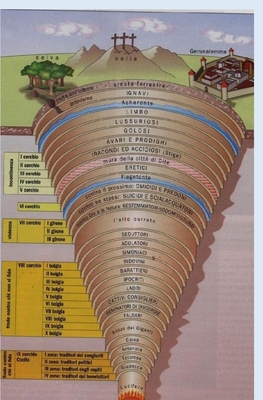
We have introduced the Divine Comedy divided into Hell, Purgatory and Paradise. It represents one of the greatest works of world literature.
It’s Dante's imaginary journey that starts from the fear in a dark forest to reach the earthly Paradise purified. Allegorically the poem represents the soul's journey towards God.
The Hell has the shape of a cone while Paradise is formed by the heavens.
Then we gave the children a task: a Dante Identity Card to fill in and a code scheme to make Dante's face.



GREECE:
ROMANIA:
PORTUGAL:
Air circulation in the atmosphere
Our Greek presentations wanted to know the Convection currents. The Earth's atmosphere is a layer of air (a mixture of gases) that surrounds the planet. The irregular warming of the Earth's surface by the Sun has implications for the climate.
In the intertropical zone, temperatures are high all year round, while in the Polar Regions the temperature is lower (usually negative).
The thermal equilibrium of planet Earth results from energy transfers in latitude.
The temperature of the atmosphere is higher over the Equator and lower at the Poles, this causes hot air at the equator which will rise.
As the mass of hot air moves to higher altitudes, it cools down and then the air becomes denser/heavier and begins to descend back to Earth, forming circulating cells.
This dynamic, associated with latitude variations, causes the wind.
Winds are a very important element in climate change. For example, in the Northern Hemisphere, the Southern wind brings up warm air from Mexico and the Caribbean to the North, while the Northern wind brings down the icy air of the Arctic region.
Wind patterns between the Equator and the polar regions would show only one direction, North or South, if it wasn't for the Earth's rotational movement.
The Coriolis Effect, a result of the Earth's rotational movement, causes winds to divert.
Surface winds are diverted to the right in the Northern Hemisphere and to the left in the Southern Hemisphere.
The formation of these meteorological phenomena is related to the movement of air masses and differences in atmospheric pressure.
Weather phenomena, such as storms, hurricanes and tornadoes, are related to differences in atmospheric pressure, humidity and temperatures at certain times of the year. So we went to the Lab to simulate these currents:
Materials
- 5 Ice cubes -1 Big jar - Cold water
- 1 Little glass - Hot water - Spoon
- Dye - Aluminum foil - Elastic
- Pencil - Chronometer
Procedure:
1. We placed the ice cubes in one of the jars and filled the bottle with cold water.
2. Filled the small glass with hot water and dye at its maximum capacity.
3. We covered the small glass with aluminum foil and fixed it with an elastic band.
4. We put the small glass inside the empty large bottle.
5. Removed the ice cubes from the other jar, and added the icy water, filling it up to 3/4.
6. We used the tip of the pencil to make a small hole in the aluminum.
7. We observed the contents of the jar for about 5 seconds.
8. We made a second hole in the aluminum.
9. We re-observed the contents of the bottle.
10. We continued to observe the contents of the vial every 5 minutes for approximately 30 minutes.
Observations
We observed the hot water rise and as it cooled down. (We could observe this movement because we put dye in the water.)
Hot water and cold water never mixed.
Conclusions
We can conclude that the less dense fluids (water or air) rise and the denser ones descend, not mixing, or mixing very slowly. So, a movement is created which we call the Convection Currents.



C5-Geography.pptx
POLAND:
And let’s move on to C5 in Volos in Greece focusing on experiments.
It was originally scheduled for April 2020.
The team of 4 boys were getting ready for the visit preparing their social experiment and constructing the “lightsaber”.
The tickets to Athens had been bought when all our lives were stopped by the Covid-19 pandemic in March 2020.
We still had hope for a visit to Greece, the project was extended a few times but eventually we could only meet on-line.
Even though it was virtual, we all have agreed that it was a success.
The Polish team grew to 11 participants - and aprt from originally planned presentations, they prepared another social experiment on how well the Poles know basic geography. Enjoy the presentations!
A social experiment on how Poles know languages:
A research project on how well Poles know basic geographical facts:
How to construct a “lightsaber”: