First of all, we had some practice with pH Universal Indicator. It is almost difficult to drop the exact quantity of Indicator to turn the solution in different pH range.
We use the comparative table below:
| pH range |
Description |
Colour |
| < 3 |
Strong acid |
Red |
| 3–6 |
Weak acid |
Orange or yellow |
| 7 |
Neutral |
Green |
| 8–11 |
Weak base |
Blue |
| > 11 |
Strong base |
Violet or purple |
The colours from yellow to red indicate an acidic solution, colours light blue to dark blue indicate bases and green colour indicates that a solution is neutral.
As show in the following video, at the beginning the solution was Strong Acid and turned to Weak Base at the end of the mixing:
PART A - HOW HARDNESS OF WATER AFFECTS THE EFFECTIVENESS OF SOAP
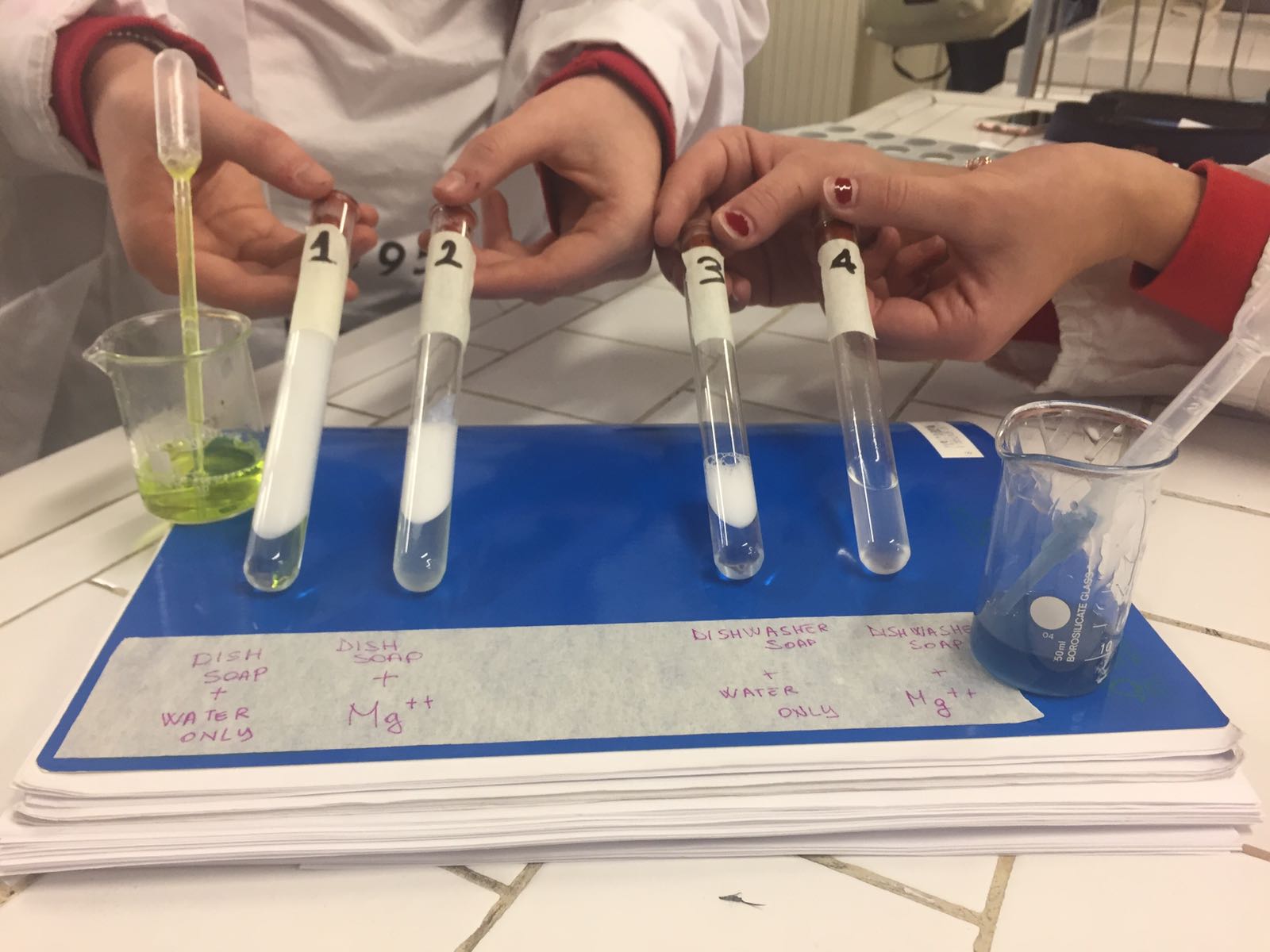
The yellow substance is dish soap, the blue one is dishwasher soap.
Although soap is a good cleaning agent, its cleaning capacity is reduced when used in hard water.
Hardness of water is due to the presence of sulphates, chlorides or bicarbonate salts of Ca2+ or Mg2+ ions.
When soap is added to hard water, the Ca2+ and Mg2+ ions present in hard water react with soap. The sodium salts present in soaps are converted to their corresponding calcium and magnesium salts which are precipitated as scum and so the cleaning capacity of soap is reduced.



PART B - QUANTITATIVE DETERMINATION OF HARDNESS IN WATER
When we went in Maremma, Tuscany we sampled several kind of water:
- Freshwater from River Ombrone
- Transitional water from Orbetello Lagoon / Delta of Ombrone
- Sea water from Tirrenian Sea / Maremma's Coast
but we were interested in know better the water we usually drink, provided by the vending machine.
Our results must be interpretated with te support of other information: the geology of the watershed or drainage basin, the pH alteration by humic acid and the Salinity.
-